Date: 2023-12-01
TAIPEI, TAIWAN──For the first time, it is possible to follow, step-by-step and with atomic resolution, what happens when the enzyme DNA photolyase repairs ultraviolet light-induced DNA damage. An international team of researchers led by Academia Sinica, Taiwan, has taken snapshots of the repair process with extremely high time resolution and combined them into a film sequence. The research was published in the scientific journal Science on December 1, 2023.
There are many types of DNA damage, which leads to diseases such as cancer or inheritable genetic disorders. The human body has evolved different processes to repair different types of damage. The present work involves the repair of solar radiation-induced thymine dimers catalyzed by the enzyme photolyase.
XFEL technology provides snapshots of the repair process at extremely high time resolution
With recent developments in X-ray crystallography and cryogenic electron-microscopy (cryo-EM), the structures of many enzymes and enzyme complexes have been determined at atomic resolution. However, a major limitation of these methods is that they only capture single pictures of the reaction, often at the beginning or the end. Unlike these technologies, X-ray Free-electron Laser (XFEL) technology can act as an extreme resolution movie camera.
The research team used the XFEL technology to determine the structures of intermediates over the entire damage repair process. This involved obtaining 18 frames in very short timeline: from 100 trillionth of a second to 200 millionths of a second. The 18 structures revealed the step-by-step process of the chemical reaction the enzyme uses to repair DNA, how the enzymatic residues facilitate the chemical reaction, how the enzyme relaxes after repair and, finally, how the repaired DNA returns to its normal shape.
A new era in enzymology
According to Academician Ming-Daw Tsai, this study advances a frontier area of structural biology and opens a new era in enzymology ── it is now possible to examine directly the entire process of an enzyme reaction at atomic resolution. The technology can also identify new structures of enzyme-bound reaction intermediates, providing a template for developing new drugs. When the technology becomes broadly applicable, its potential for applications is expected to be immense.
The paper has 69 coauthors from 17 research institutions worldwide. Main contributors include Academician Ming-Daw Tsai, an expert in the enzymology of DNA repair enzymes, at Academia Sinica and Professor Lars-Oliver Essen of Phillips University at Marburg, Germany, a leading structural biologist of photolyases. The first author, Dr. Manuel Maestre-Reyna, carried out much of the work while working at Academia Sinica and was recently appointed as an Assistant Professor at National Taiwan University. Other authors include Dr. Yoshitaka Bessho of Academia Sinica, currently at the University of Tokyo, Dr. Antoine Royant, a CNRS director of Research at the University of Grenoble Alps and Dr. Junpei Yamamoto at Osaka University.
The research was funded primarily through the Taiwan Protein Project, which is one of the National Science and Technology Programs carried out by Academia Sinica. The XFEL experiments were performed at SACLA, Japan and SwissFEL, Switzerland.
-
Dr. Ming-Daw Tsai, Institute of Biological Chemistry, Academia Sinica
(02) 2785-5696#3070, mdtsai@gate.sinica.edu.tw
-
Dr. Manuel Maestre-Reyna, Department of Chemistry, National Taiwan University
(02) 3366-8682, mmaestre@ntu.edu.tw
-
Ms. Wen Li Wang, Media & Public Affairs,Secretariat, Central Administrative Office, Academia Sinica
(02)2789-9727,cassiew@as.edu.tw
-
Ms. Steffi Tung Lin, Media & Public Affairs, Secretariat, Academia Sinica
(02) 2789-8820,tunglin@as.edu.tw
-
Link
-
 Academician Ming-Daw Tsai and Dr. Manuel Maestre-Reyna, Department of Chemistry, National Taiwan University. Photo credit: Academia Sinica.
Academician Ming-Daw Tsai and Dr. Manuel Maestre-Reyna, Department of Chemistry, National Taiwan University. Photo credit: Academia Sinica.
-
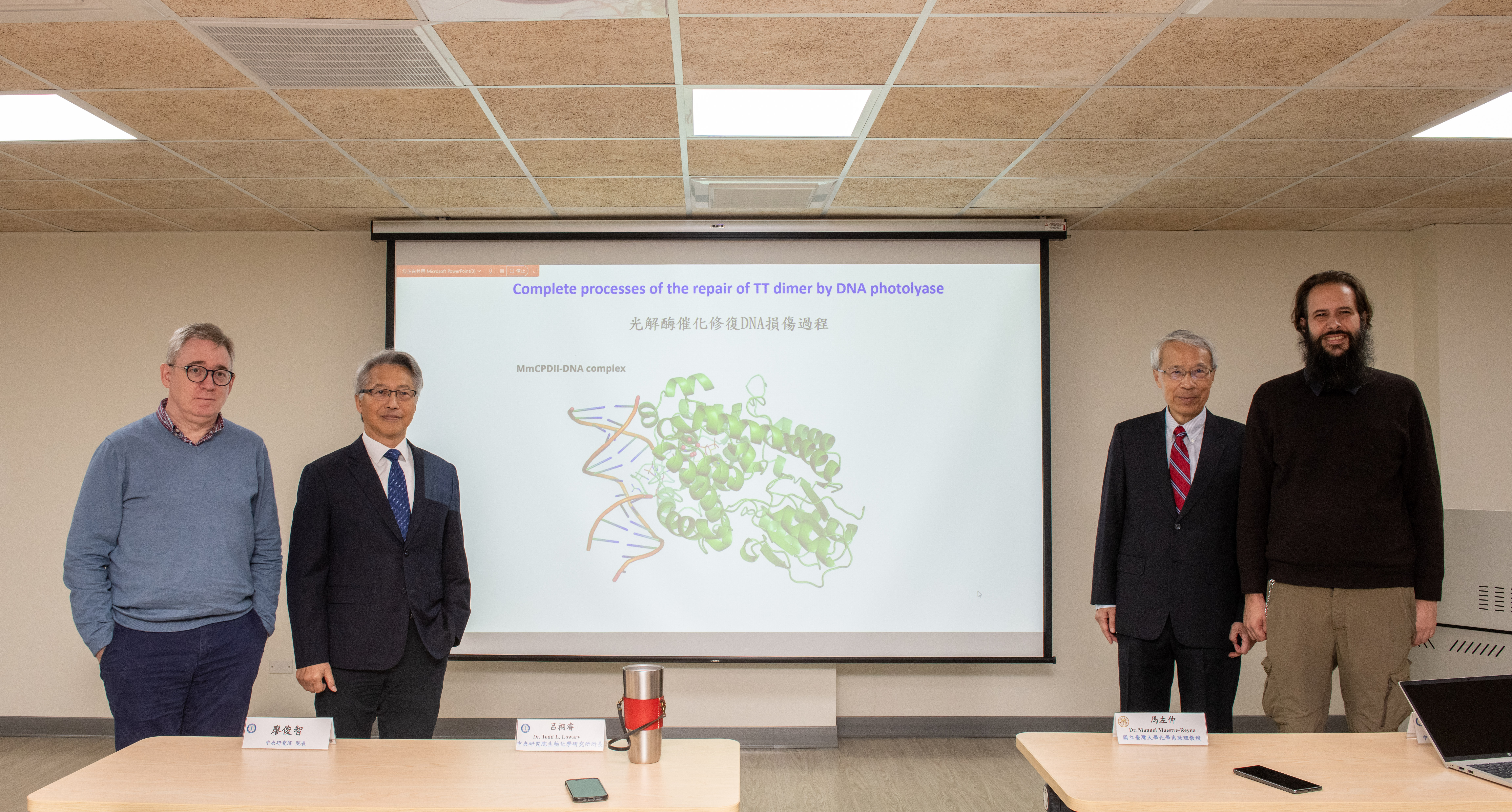 Director Todd L. Lowary, Institute of Biological Chemistry, Academia Sinica; President James C. Liao, Academia Sinica; Academician Ming-Daw Tsai, Institute of Biological Chemistry, Academia Sinica; Assistant Professor Manuel Maestre-Reyna, Department of Chemistry, National Taiwan University. Photo credit: Academia Sinica.
Director Todd L. Lowary, Institute of Biological Chemistry, Academia Sinica; President James C. Liao, Academia Sinica; Academician Ming-Daw Tsai, Institute of Biological Chemistry, Academia Sinica; Assistant Professor Manuel Maestre-Reyna, Department of Chemistry, National Taiwan University. Photo credit: Academia Sinica.
-
 Academician Ming-Daw Tsai. Photo credit: Academia Sinica.
Academician Ming-Daw Tsai. Photo credit: Academia Sinica.









 Home
Home