Date: 2018-03-01
The rise of antibiotic resistance together with the lack of effective treatment creates a major threat to human health. Wei-Shen Wu, a Ph.D. student from the National Defense Medical Center, recently uncovered a potential finding that can counter antibiotic resistance. Under the direction of Dr. Rachel Cheng and Dr. Chi-Huey Wong at the Genomics Research Center, Wei-Shen Wu identified natural products that can serve as antibacterial agents. These findings were recently published in the Journal of American Chemical Society.
Due to the rampant improper use of antibiotics coupled with global warming and increasing human contact through globalization, dangerous, antibiotic resistant bacteria have been observed with increasing frequency over the past several decades. In the absence of effective treatment, drug-resistant bacteria have created a surmounting crisis across the globe. As such, the development of new antibiotics against drug-resistant bacteria, such as Acinetobacter baumannii and Pseudomonas aeruginosa, is of utmost importance and certainly a medical need that must be met.
As we know, some antibiotics work by affecting or attacking the cell wall of bacterial cells. Bacteria build cell walls by linking molecules together; without the support from a cell wall, pressure inside the cell of the bacteria becomes too much and the membrane bursts. A major constituent of the bacterial cell wall is peptidoglycan, which serves a structural role in the bacterial cell wall. Peptidoglycan is synthesized by a bi-functional enzyme known as the penicillin-binding protein (PBP), which consists of two enzymatic domains, transglycosylase (TGase) and transpeptidase.
Understanding these structural characteristics, scientists have developed antibiotics that target either transglycosylase (TGase) or transpeptidase. To date, numerous transpeptidase-targeted antibiotics have been successfully developed. Whereas, only a few compounds have been identified to inhibit TGase, and these have yet to be developed into antibiotics for human use. In light of its accessible location and critical function, the bacterial TGase has been recently recognized as an attractive drug target to tackle the problem of antibiotic resistance.
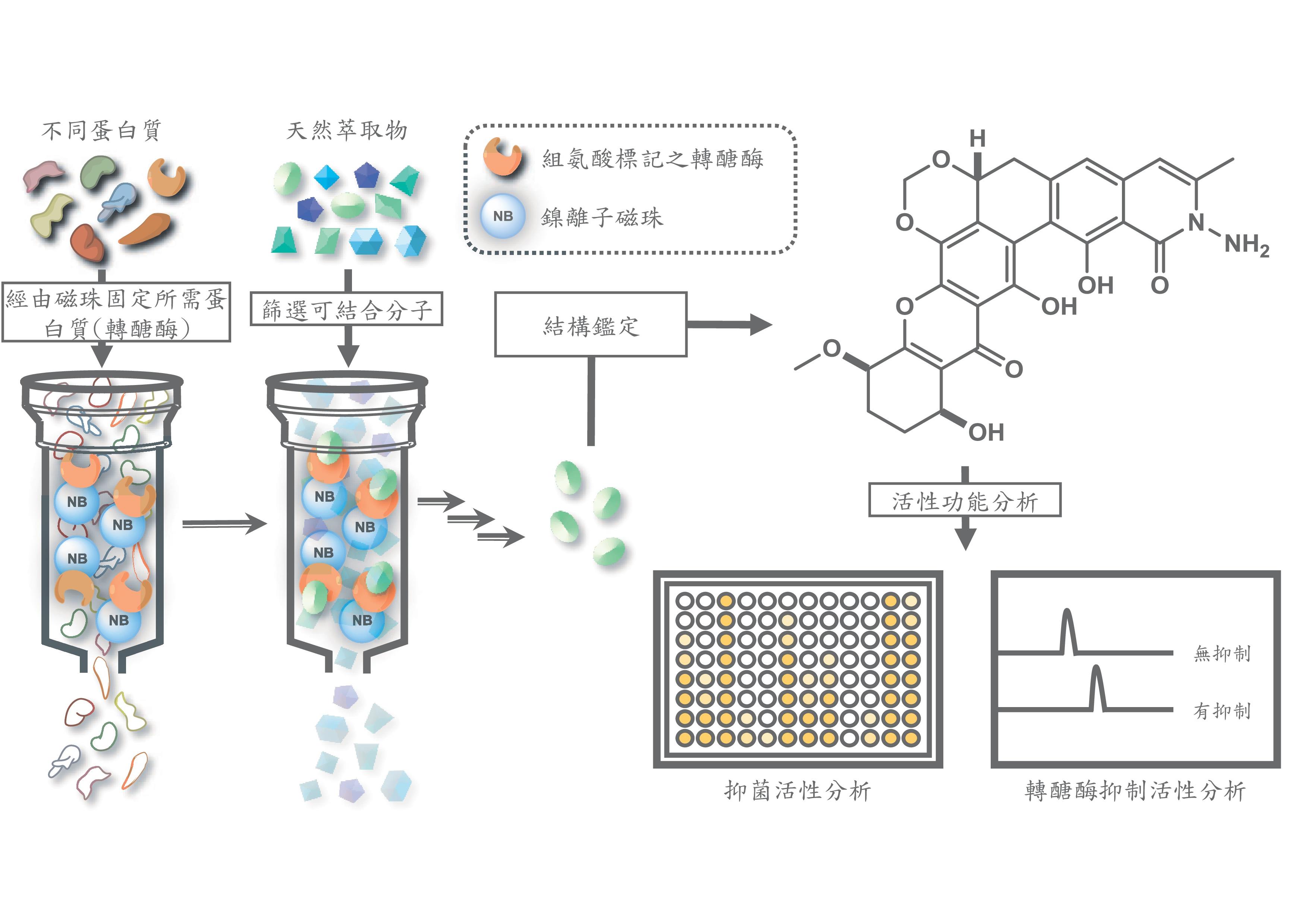
Taking advantage of this characteristic of TGase, Wei-Shen Wu and his mentors worked to develop new antibiotics that target the uncharted surface of bacterial transglycosylase. Their answer came in the form of natural products. Natural products have historically been recognized as a prolific and dependable source of antibacterial agents. Wu, leading author of the recently published article entitled “Affinity-Based Screen for Inhibitors of Bacterial Transglycosylase,” and his mentors devised a fast and effective affinity-based screen technology using immobilized TGase to selectively isolate four potent inhibitors from fermentation broths.
These four natural products or compounds—TAN1532B, Bequinostatin E, Benastatin B, and Albofungin—showed very strong antibiotic activities against a broad-spectrum of Gram-positive bacteria, including the drug-resistant strains of enterococci and staphylococci. Moreover, one of these compounds, Albofungin, was also very effective against Gram-negative bacteria such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumonia, as well as their drug-resistant mutants.
Encouraged by the potency of these natural compounds against a variety of Gram-positive and Gram-negative bacteria, the research group has initiated collaboration projects with other labs to test if the antibiotics are effective against other clinically resistant strains of bacteria. The team also aims to better understand the complex molecular structure of bacterial TGase when interacting with these natural inhibitors in order to develop better and more effective antibiotics.
-
Chang-Hung Chen, Public Affairs Section, Secretariat, Academia Sinica
(02) 2789-8059,changhung@as.edu.tw
-
Mr. Chung-Hui Chuang, Media Team, Secretariat, Central Administrative Office, Academia Sinica
(02) 2789-8820,chchuang@gate.sinica.edu.tw









 Home
Home