Date: 2025-07-18
In living cells, reactive oxygen species (ROS) play a dual role — they are important molecules that regulate cellular metabolism and signaling, but excessive ROS can lead to oxidative damage and chronic inflammation. For years, it has been widely recognized that oxidative stress is closely associated with immune imbalance, aging, cancer, and metabolic diseases. However, due to the lack of high-resolution tools to dynamically analyze redox signaling, the fundamental question of “how redox signals drive immune cell fate” has remained unresolved.
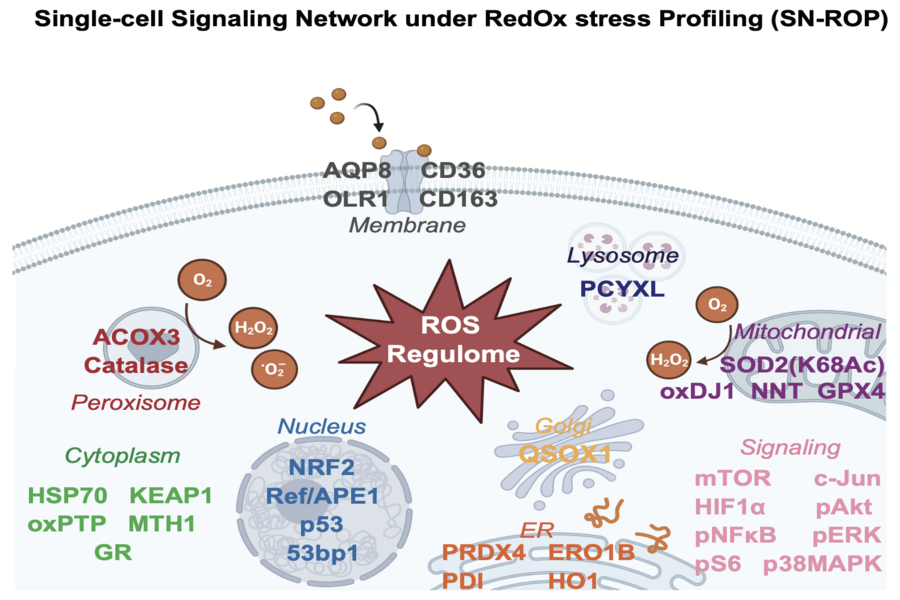
To address this, the research team developed an innovative technique called Single-cell Redox Signaling Network Profiling (SN-ROP). This method leverages mass cytometry (CyTOF) with metal-labeled antibodies to simultaneously quantify multiple redox indicators and signaling activities at the single-cell level. This allows researchers to track immune cell responses and fate transitions under oxidative stress.
The SN-ROP approach was applied to both mouse and human samples. The team discovered that early redox balance is closely linked to protein folding and cellular stress signaling. Disruption of this balance can push immune cells into distinct fate trajectories. Further analysis revealed coordinated redox signaling in CD8⁺ T cells upon antigen stimulation. The method also successfully identified oxidative signatures associated with CAR-T cell persistence and immune dysfunction in dialysis patients. These findings demonstrate that SN-ROP is a powerful tool for exploring redox regulatory networks in the immune system and may have broad applications in chronic diseases, cancer, and immunotherapy development.
This study was led by Dr. Shih-Yu Chen, Academia Sinica, and conducted with his PhD student Yi-Chuan Wang, as well as research teams from National Taiwan University Hospital, Kaohsiung Medical University, and University of Lausanne in Switzerland. The research was published on July 1, 2025, in Nature Communications.









 Home
Home