Date: 2025-10-16
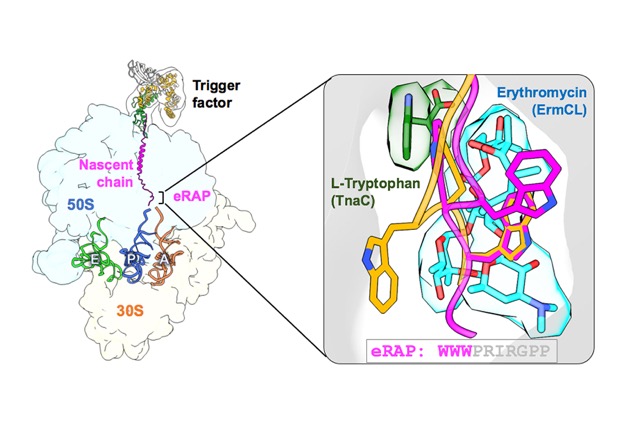
A research team led by Dr. Shang-Te Danny Hsu at the Institute of Biological Chemistry, Academia Sinica, has used high-resolution cryo-electron microscopy to reveal how an artificially designed ribosome arrest peptide (eRAP) precisely pauses the ribosome during protein synthesis. This temporary pause helps the cell control both the timing and quality of protein production.
The research team found that eRAP combines features from two natural ribosome stalling systems — TnaC, which responds to the amino acid tryptophan, and ErmCL, which reacts to the antibiotic erythromycin. Together, these elements form a structure that more effectively halts the ribosome during protein translation. Through cryo-electron microscopy structural analysis, the team showed that eRAP makes specific contacts with ribosomal RNA and proteins inside the ribosome’s tunnel, triggering conformational changes that lead to translational pausing. The study also revealed that the newly synthesized protein interacts with the chaperone Trigger Factor near the ribosome exit site, influencing its early folding to form a longer helical structure during the initial stages of synthesis. This discovery not only unveils the molecular mechanism that allows the ribosome to “pause,” but also provides new insights for precisely controlling protein synthesis and developing next-generation antibiotics.
The study is supported by Academia Sinica with the technical supports from the Academia Sinica Cryo-EM Center. The first author, Dr. Manoj Kumar Sriramoju, is a postdoctoral fellow funded by the National Science and Technology Council, Taiwan. The study has been published in Nucleic Acids Research on 15 October 2025.
-
Link









 Home
Home