Date: 2020-11-16
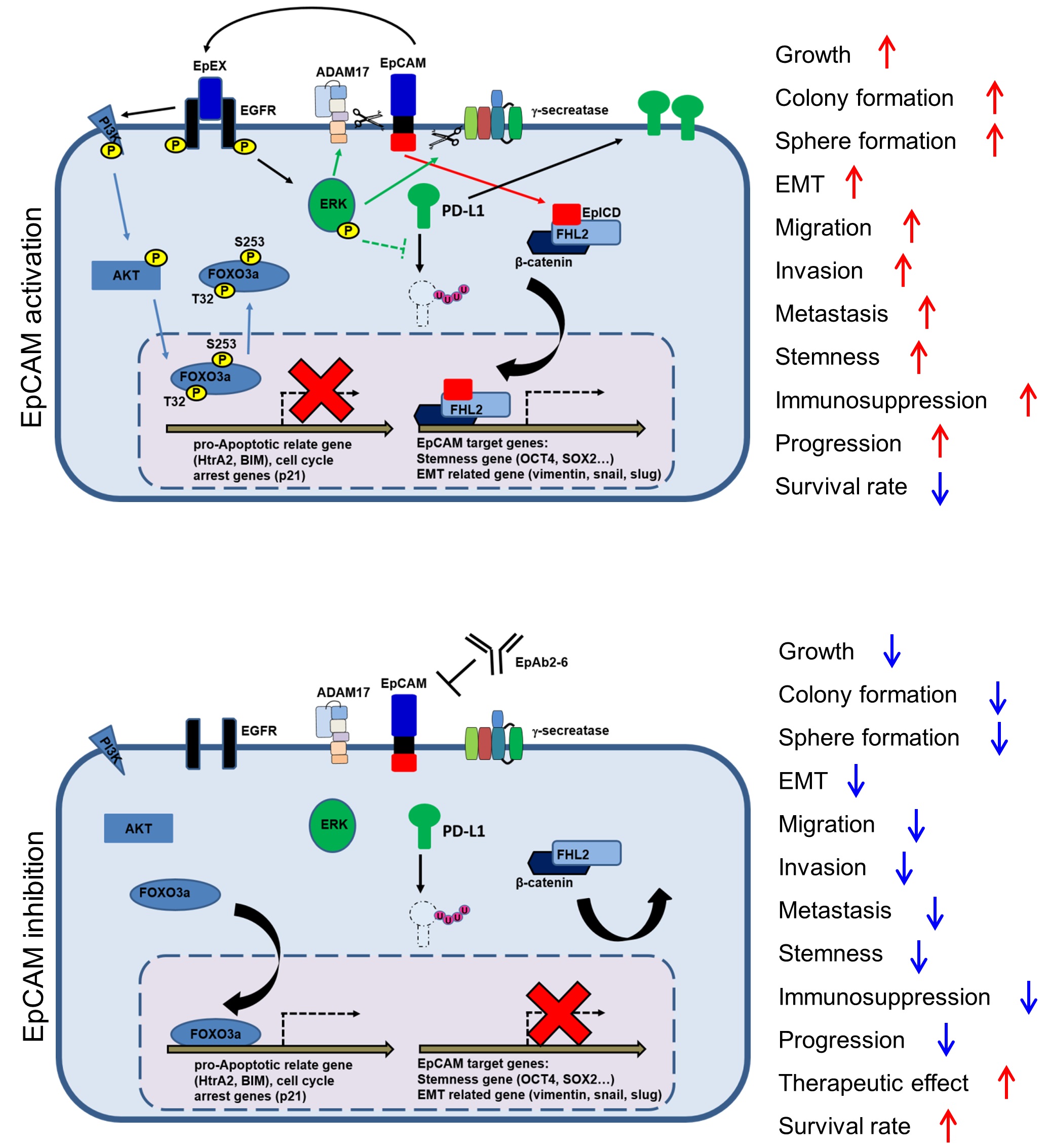
In the world of cutting-edge cancer research, the cell-surface molecule EpCAM is widely thought of as a promising target for new cancer diagnostics and therapeutics. A recent study by Dr. Han-Chung Wu’s group at the Institute of Cellular and Organismic Biology, Academia Sinica, has greatly advanced our knowledge of how EpCAM acts to promote cancer cell growth and how that process can be stopped. The study was published in the world-renowned journal, Cancer Research 1, and showed that EpCAM acts through growth factor signaling to stabilize another cell-surface molecule, PD-L1, which allows tumor cells to escape recognition by the patient’s immune system.
Most importantly, a new neutralizing antibody tool developed by Wu’s research group was able to prevent EpCAM signaling and decrease the level of PD-L1 in cancer cells, which led to cancer cell death through this and other mechanisms. Other methods for decreasing PD-L1 action have proven quite effective in the clinic. Based on this clinical success, immune checkpoint inhibitors like CTLA4 and PD-1 earned the Nobel Prize in Physiology or Medicine in 2018. Since EpCAM is enriched in many types of cancer cells, the findings and tools from this study have the potential to broadly impact cancer diagnosis and treatment.
The neutralizing antibody in this report is called EpAb2-6, and it is patent-protected in many countries around the world. Recently, the group obtained NT$50 million funding from the TRUST-U (Taiwan Reputed University Start-ups to Taiwan Unicorns) program to be used for therapeutic antibody drug development. Using this funding, they further established a biomedical start-up company at the National Biotechnology Research Park that can bridge the gap between basic science and clinical application.
Dr. Wu’s laboratory has studied the EpCAM molecule for many years and published several reports in top journals on the cellular mechanisms of EpCAM-mediated tumorigenesis and development of neutralizing antibodies. In a series of ground-breaking studies, they previously learned that EpCAM (which stands for Epithelial Cell Adhesion Molecule) is an important factor that can participate in converting cells into to a stem cell state. The group first found that EpCAM is highly and selectively expressed in undifferentiated human embryonic stem cells (hESC) rather than differentiated hESCs 2. They then went on to discover that the intracellular domain of EpCAM (EpICD) directly regulates several stem cell reprogramming genes, including c-MYC, OCT-4, NANOG, SOX2, and KLF4, to help maintain the undifferentiated state of hESCs 2. While the Nobel laureate, Shinya Yamanaka, succeeded in reprogramming fibroblasts into pluripotent stem cells (iPSCs) using the four Yamanaka factors (OCT-4, SOX2, KLF4, c-MYC), Dr. Wu’s group was able to accomplish the same feat using only EpCAM and a single Yamanaka factor, either Oct4 or Klf4 3. Moreover, the group found that the extracellular domain of EpCAM (EpEX) enhances the multipotency and proliferation of mesenchymal stem cells through EGFR-LIN28-LET7 signaling, and it promotes osteogenesis of MSCs under differentiation conditions 4. These findings unveil new strategies for iPSC formation and maintenance of stemness that may have direct application in regenerative medicine.
Based on its essential role in many cancers, EpCAM has become widely known as a potential target for diagnotics and antibody-based immunotherapies for malignancies. Therefore, Dr. Wu’s group used hybridoma technology to generate mAbs against EpCAM, including one with particularly high affinity to SAS, HCT116 and AsPC-1 cells, called EpAb2-6 5. Strikingly, EpAb2-6 was found to directly induce apoptosis of cancer cells in vitro 5-7. Hence, Dr. Wu’s group further used phage display and site-directed mutagenesis to identify the exact B cell epitope of EpAb2-6, which turned out to be Y95 and D96 in the EGF-II/thyroglobulin (TY) domain 5,7. In additon, they found that EpAb2-6 inhibits the nuclear translocation of EpICD with β-catenin and prolongs overall survival in both pancreatic cancer metastasis and human colon carcinoma xenograft mouse models 5,6. Since EpAb2-6 is a high affinity antibody and the first anti-EpCAM antibody with the ability to directly trigger apoptosis in cancer cells, it can be used not only for cancer diagnosis and imaging, but also for targeted therapy. The antibody has been patented in many countries, and work is underway to develop its applications.
In the most recent study, Dr. Wu’s group found the EpCAM also participates in tumor progression and immunosuppression 1. They reported that the EGF-like domain I within the EpCAM extracellular domain (EpEX) binds EGFR, activating both AKT and MAPK signaling to respectively inhibit FOXO3a function and stabilize PD-L1 protein. Treatment with the EpCAM-neutralizing antibody, EpAb2-6, inhibited AKT and FOXO3a phosphorylation, increased FOXO3a nuclear translocation, and upregulated HtrA2 expression to promote apoptosis, while decreasing PD-L1 protein levels to enhance the cytotoxic activity of CD8+ T cells. In vivo, EpAb2-6 markedly extended survival in mouse metastasis and orthotopic models of human colorectal cancer. The combination of EpAb2-6 with Atezolizumab, an anti-PD-L1 antibody, almost completely eliminated tumors. Moreover, the number of CD8+ T cells in combination-treated tumors was increased compared to Atezolizumab alone. The use of immune checkpoint inhibitors, such as monoclonal antibodies against CTLA-4, PD-1 and PD-L1, represents a major breakthrough in cancer treatment. These therapeutics can greatly extend the life of cancer patients and make many previously incurable cancers curable. Therefore, the recent findings suggest an exciting new combined treatment strategy for cancer immunotherapy in patients with EpCAM-expressing tumors.
The studies by Dr. Han-Chung Wu and his group on the cellular mechanisms of EpCAM-mediated tumorigenesis and the development of neutralizing antibodies were all published in top journals. There are five major innovations that came from this research: (1) EpCAM/EpEX activates EGFR signaling (2) FOXO3a regulates HrtA2 (3) EpCAM/EpEX regulates PD-L1 protein stability (4) Novel anti-EpCAM therapeutic antibodies were identified for cancer research (5) EpCAM can successfully reprogram fibroblasts into iPSCs along with a single Yamanaka factor, Oct4 or Klf4. Based on these innovations and through their new company, Dr. Wu’s group is poised to create new and effective treatments for cancer.
References
1 Chen, H. N. et al. EpCAM signaling promotes tumor progression and protein stability of PD-L1 through the EGFR pathway. Cancer Res, Accepted (2020).
2 Lu, T. Y. et al. Epithelial cell adhesion molecule regulation is associated with the maintenance of the undifferentiated phenotype of human embryonic stem cells. J Biol Chem 285, 8719-8732, doi:10.1074/jbc.M109.077081 (2010).
3 Kuan, II et al. EpEX/EpCAM and Oct4 or Klf4 alone are sufficient to generate induced pluripotent stem cells through STAT3 and HIF2alpha. Sci Rep 7, 41852, doi:10.1038/srep41852 (2017).
4 Kuan, II et al. The extracellular domain of epithelial cell adhesion molecule (EpCAM) enhances multipotency of mesenchymal stem cells through EGFR-LIN28-LET7 signaling. J Biol Chem 294, 7769-7786, doi:10.1074/jbc.RA119.007386 (2019).
5 Liao, M. Y. et al. An anti-EpCAM antibody EpAb2-6 for the treatment of colon cancer. Oncotarget 6, 24947-24968, doi:10.18632/oncotarget.4453 (2015).
6 Liang, K. H. et al. Extracellular domain of EpCAM enhances tumor progression through EGFR signaling in colon cancer cells. Cancer Lett 433, 165-175, doi:10.1016/j.canlet.2018.06.040 (2018).
7 Liao, M. Y., Kuo, M. Y., Lu, T. Y., Wang, Y. P. & Wu, H. C. Generation of an anti-EpCAM antibody and epigenetic regulation of EpCAM in colorectal cancer. Int J Oncol 46, 1788-1800, doi:10.3892/ijo.2015.2876 (2015).
-
Chang-Hung Chen, Public Affairs Section, Secretariat, Academia Sinica
(02) 2789-8059,changhung@as.edu.tw
-
Mr. Chung-Hui Chuang, Media Team, Secretariat, Central Administrative Office, Academia Sinica
(02) 2789-8820,chchuang@gate.sinica.edu.tw









 Home
Home