Date: 2017-10-25
In the battle against cancers, more and more attention is shifted toward the cells around the cancer cells, which is the microenvironment, including fibroblast cells, immune cells, and fat cells. The research team led by Dr. Wen-Hwa Lee, an Academia Sinica academician, the Distinguished Research Fellow of Academia Sinica and the Chancellor of Chinese Medical University has recently identified a mechanism for how breast cancer cells thrive in a microenvironment full of regulatory T cells and adipose tissue, and provide new information for breast cancer therapy.
Lee’s team took the initiative and came to the conclusion that tumor-draining lymph nodes (TDLNs) play a crucial role in breast cancer malignancy. Researchers concluded that the depletion of Tregs in TDLNs would end oncogenic IL-17RB induction as well as metastatic activities in breast cancer cells.
It is very exciting to see that the removal of TDLNs at early stages would indeed reduce migrations of breast cancer cells to distant organs. In addition, the pursuit of developing IL-17RB antibodies looks promising for preventing breast cancer metastasis. The research achievement has been published in EMBO Molecular Medicine on October 9, 2017.
When breast cancer cells are found in the lymph nodes, it is considered an important prognostic predictor for patient survival. However, whether TDLNs take part in the course of breast cancer metastasis has never been thoroughly examined. According to Dr. Shih-Chia Huang, the first author of the research publication of this study, axillary lymph node dissection has been adopted for melanoma therapeutics and provided favorable prognosis, while in terms of breast cancer, this procedure is mostly for the determination of the stage of cancer.
One would think that cancer cells should avoid lymph nodes to protect themselves from the immune system. However, Lee’s team noted that breast cancer cells derived from TDLNs showed a significantly higher metastatic ability. In reality, they have discovered that Treg cells, which are supposed to guard abnormal immune behaviors, would secrete a molecule called TGF-β1. TGF-β1 up-regulates IL-17RB expression, and the IL-17RB, which has been a target for cancer study in Lee’s lab, is the gene that makes breast cancer cells stronger to migrate to distant organs like the lungs.
Besides the cardiovascular system, which distributes blood, there is the lymphatic system that circulates lymph. Together, they form the circulatory system, which is the transportation system that allows for the interchange of substances to take place in our body. The lymphatic system is connected by lymphatic vessels. Compared to blood vessels, lymphatic vessels are porous and easily accessed by cells. Lymphatic vessels take advantage of their much lower flow rate to increase the survival of cells by minimizing shear stress. Therefore, for cancer cells that long for a journey to other organs, lymph vessels are ideal channels.
Lymph nodes are like checkpoints throughout the lymphatic system, which has all sorts of immune cells on duty to guard our body, and the TDLNs, which connect tumors, are no exception. However, the team demonstrated in a series of animal tests that inside TDLNs is a kind of immune cell known as Tregs (regulatory T cells) that actually enhances breast cancer malignancy.
In another research, Lee’s team indicated that, besides immune cells, fat cells are also responsible for providing a nourishing environment to trigger the breast cancer cells to multiply.
For breast cancer, clinical observations indicated higher risks for women with larger breasts as well, and poor prognosis seemed to happen more to clinical cases when breast cancer cells had already invaded into the fatty tissues. All these clues encouraged Lee’s team to look into it.
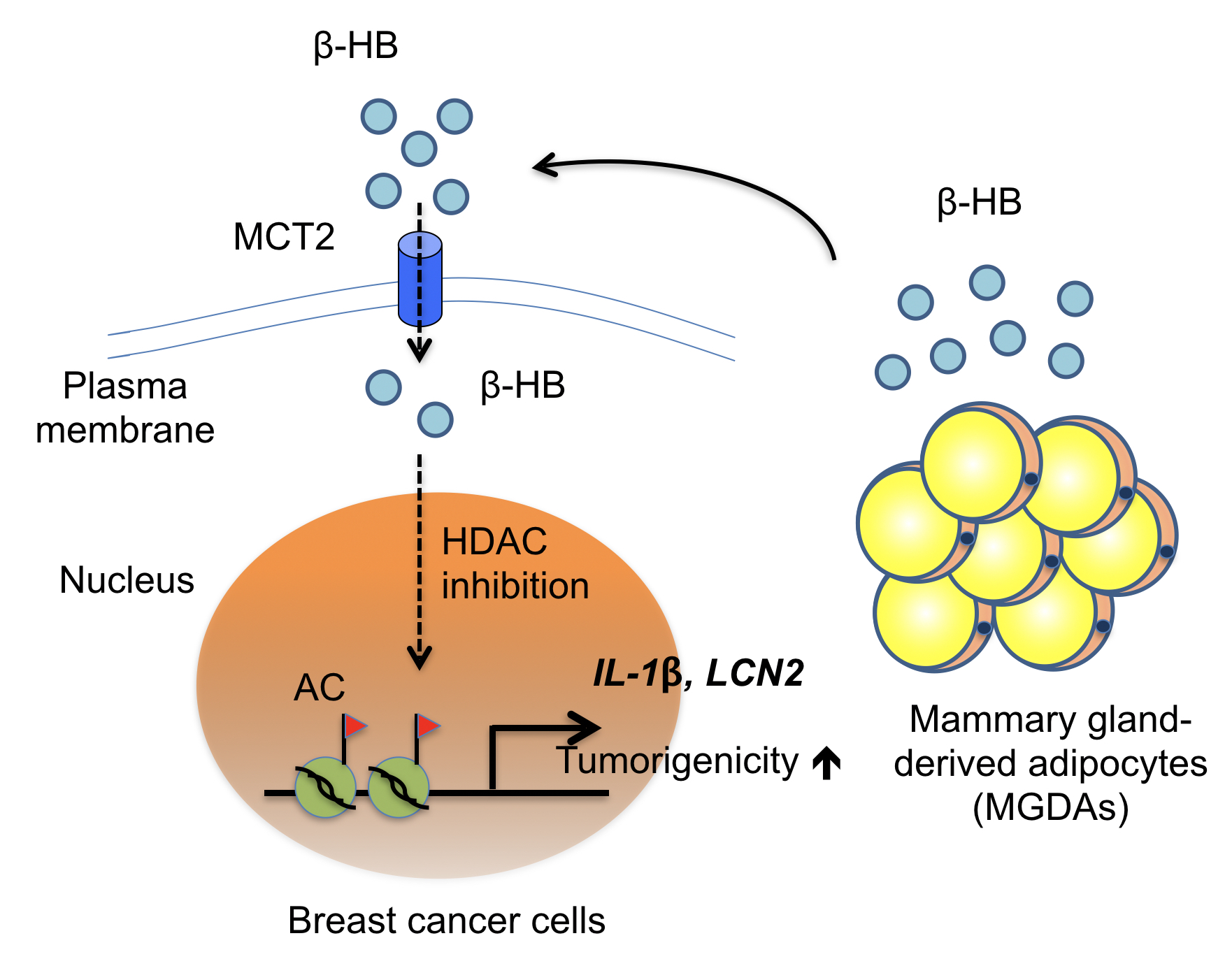
First author Dr. Chun-Kai Huang in this case emphasized that, unlike known studies, Lee’s team decided to study this cancer/fat cell interaction using the fatty tissue biopsy derived from breast cancer patients. Through a co-culture method growing layers of fatty tissues with various breast cancer cell lines, the research team identified a gene called monocarboxylate transporter 2, also known as MCT2, which plays a crucial role in this cancer/fat culture model.
Prior research has described MCT2 as a transporter on the cancer cell membrane allowing in-and-outs of beta-hydroxybutyric acid (β-HB), which was also proved to be another determining factor for MCT2-expressing breast cancer progression in this study. In the neighborhood of breast cancer cells, MCT2 will keep the supply of β-HB derived from fatty tissues for breast cancer nourishment. Therefore, MCT2 can be a target to find therapeutics down the road!
These studies were done by Dr. Wen-Hwa Lee’s team at the Genomics Research Center at Academia Sinica in collaboration with physicians from National Taiwan University hospitals such as Dr. Chiun-Sheng Huang, Dr. King-Jen Chang, Dr. Wen-Hung Kuo, and Dr. Yung-Ming Jeng. The papers entitled “TGF-β1 Secreted by Tregs in Lymph Nodes Promotes Breast Cancer Malignancy via Up-regulation of IL-17RB” and “Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate” have been published in EMBO Molecular Medicine on October 9, 2017 and Nature Communications on March 10, 2017, respectively.
Website: “TGF‐β1 secreted by Tregs in lymph nodes promotes breast cancer malignancy via up‐regulation of IL‐17RB” http://embomolmed.embopress.org/content/early/2017/10/09/emmm.201606914
Website: “Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate” https://www.nature.com/articles/ncomms14706.
01: Schematic diagram of Tregs secreting TGF-β1 in TDLNs to promote breast cancer malignancy via up-regulation of IL-17RB.
02: Heterotypic interaction between MGDAs and MCT2-expressing breast cancer cells in the breast tissue microenvironment
-
Ms. Shih-Wen Huang, Secretariat, Academia Sinica
(02) 2789-9868,shihwen@as.edu.tw
-
Chang-Hung Chen, Public Affairs Section, Secretariat, Academia Sinica
(02) 2789-8059,changhung@as.edu.tw









 Home
Home