Date: 2024-03-28
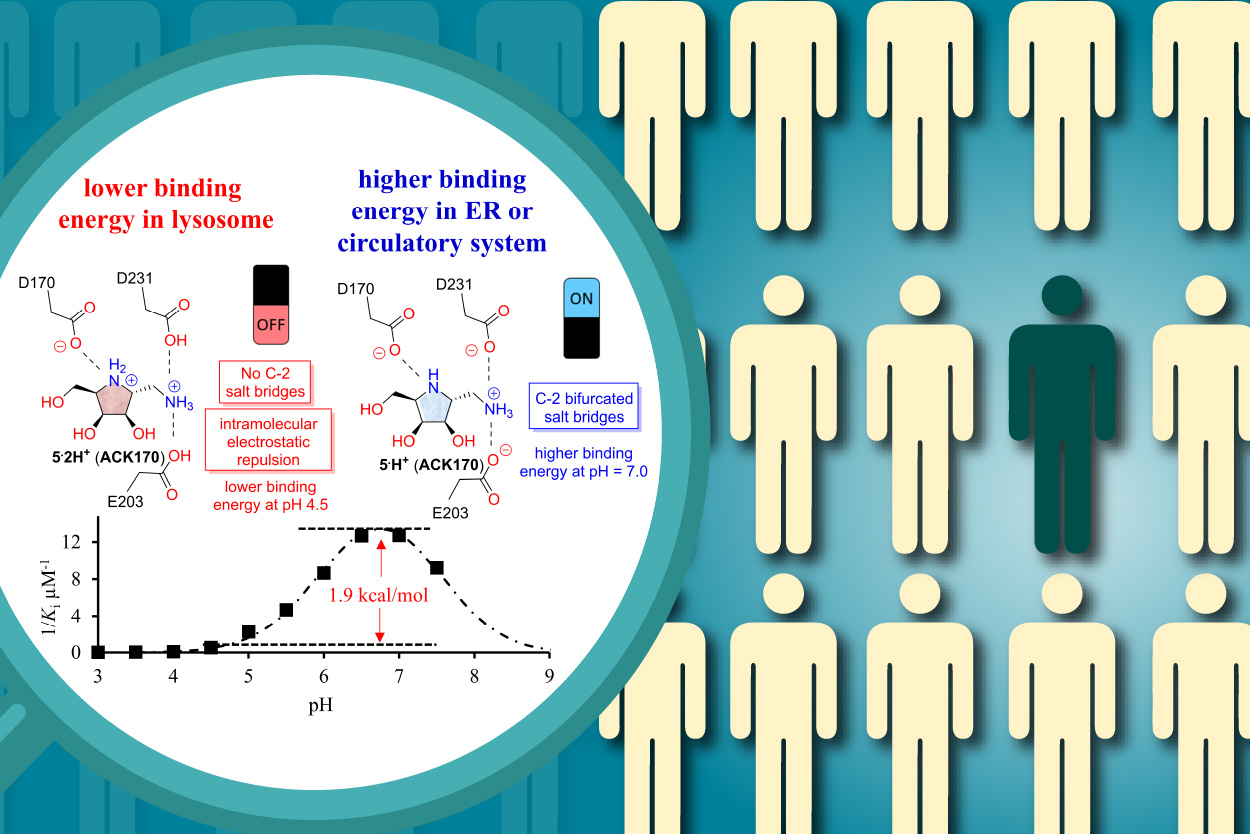
The use of protein stabilizers or pharmacological chaperones to protect specific enzymes and impart a therapeutic benefit is an emerging strategy in drug discovery. However, designing molecules that can bind optimally to their targets at physiological pH remains a major challenge. The research, led by Dr. Wei-Chieh Cheng at Genomics Research Center, Academia Sinica, found that a dibasic polyhydroxylated pyrrolidine ACK170 (US 10995067 B2) exhibited superior pH-selective inhibitory activity and chaperoning activity for human α-galactosidase A (α-Gal A) through the Natural Product-Inspired Combinatorial Chemistry (NPICC) strategy. To further investigate the role of different C-1 moieties on the pH-selectivity and protecting effects of these compounds, researchers designed and synthesized a library of monobasic and dibasic iminosugars, screened them for α-Gal A stabilizing activity using thermal shift and heat-induced denaturation assays, and characterized the mechanistic basis for this stabilization using X-ray crystallography and binding assays. It was noted that the dibasic iminosugar ACK170 protect α-Gal A from denaturation and inactivation at lower concentrations than monobasic or other N-substituted derivatives; a finding attributed to the nitrogen on the C-1 methylene of ACK170 which forms the bifurcated salt bridges with two carboxyl residues, E203 and D231, and is responsible for their pH-selective binding to α-Gal A. Moreover, ACK170 demonstrated promising results to improve enzyme replacement therapy and exhibited significant chaperoning effects in Fabry cells. These findings suggest that amino-iminosugar ACK170 is a useful model to demonstrate how an additional exocyclic amino group can improve their pH-selectivity and protecting effects. This research has been published on February 23, 2024 in JACS Au, providing new insights into the design of pH-selective small molecules for the treatment of lysosomal storage diseases.
-
Link









 Home
Home